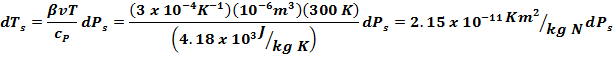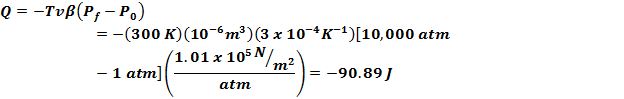|
|
|
|
P 6 – 26 The isothermal
compressibility of water is 50 x 10-6 atm-1 and cP
= 4.18 x 103 J kg-1 K-1. Other properties of water are given in the
previous problem. Calculate the work
done as the pressure on 1 g of water in a hydraulic press is increased
reversibly from 1 atm to 10,000 atm (a) isothermally, (b) adiabatically, (c)
Calculate the heat evolved in (a) the isothermal process. |
|
|
|
|
|
Calculate
the work done as the pressure on 1 g of water in a hydraulic press is
increased reversibly from 1 atm to 10,000 atm (a) isothermally, |
|
|
|
|
|
From
section 6-9 we are reminded that dv can be written as |
|
|
|
|
|
|
|
|
So
work can be found from |
|
|
|
|
|
Isothermal
reduces this to |
|
|
|
|
|
|
|
|
|
|
|
From
problem 6-25, if we assume the temperature is 4°C, then v = 1 cm3
= 10-6 m3 |
|
|
|
|
|
|
|
|
So
work per gram is |
|
|
|
|
|
|
|
Calculate
the work done as the pressure on 1 g of water in a hydraulic press is
increased reversibly from 1 atm to 10,000 atm (b) adiabatically, |
|
|
|
|
|
Again
work is found from |
|
|
|
|
|
We
need to find dT for adiabatic, from eq (6-34) |
|
|
|
|
|
|
|
|
But
β = 3 x 10-4 K-1 for water at 300 K, so |
|
|
|
|
|
|
|
|
Compared
to dPs, we can ignore this term. So work can now be found from |
|
|
|
|
|
|
|
|
We
now must replace k with ks = k/g |
|
|
|
|
|
|
|
|
Eq
(6-12) gives us |
|
|
|
|
|
So
cP = cv and g = 1 so ks = k, so the work adiabatically is the
same as the work isothermally. |
|
|
|
|
|
|
|
(c)
Calculate the heat evolved in (a) the isothermal process. |
|
|
|
|
|
Eq
(6-31) |
|
|
|
|
|
Since
isothermal dT = 0 |
|
|
|
|
|
Integrating |
|
|
|
|
|
Thus
the heat evolved isothermally is |
|
|
|
|
|
|
|
|
|
|
|